A research group led by Associate Professor Takashi Tachikawa of Kobe University’s Molecular Photoscience Research Center has developed a strategy that greatly increases the amount of hydrogen produced from sunlight and water using hematite (α‐Fe2O3) photocatalysts.
They were able to raise the conversion rate up to 42% of its theoretical limit (16%) by synthesizing tiny nanoparticle subunits in the hematite. A paper on their work appears in the journal Angewandte Chemie International Edition.
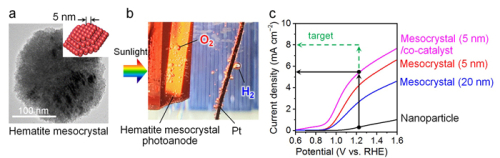
Mesocrystal photoanode formation and photochemical water splitting characteristics. a. Electron microscope image of a hematite mesocrystal (assembled from tiny nano-particles of approx. 5nm). b. Gas production from the anode. c. Graph to show the current density and applied voltage. The anode is the photocatalyst anode, and a platinum electrode was used for the cathode. The potential is based on the RHE (Reversible Hydrogen Electrode). The oxidation potential is 1.23V. The solar water splitting capacity was greatly enhanced by making the nano-particles in the mesocrystal structures smaller. Zhang et al.
Hematite is a type of iron oxide ore. In addition to being safe, inexpensive and stable (pH > 3), Hematite can absorb a wide range of visible light (approx. under 600nm). The theoretical limit of its solar energy conversation efficiency is 16% (a photocurrent density of 13 mA cm-2).
Tachikawa and his colleagues successfully produced a photoanode with an extremely high conductivity by annealing hematite mesocrystals (superstructures consisting of tiny nanoparticles of approx. 5nm) to a transparent electrode substrate.
Numerous oxygen vacancies were formed inside the hematite mesocrystals by accumulating and sintering tiny highly-orientated nanoparticles of less than 10 nanometers.
Inside the mesocrystal structure, there are spaces where there is no oxygen—i.e., oxygen vacancies (Vo). In hematite, the creation of these oxygen vacancies enhances electrical conductivity because Fe3+ is deoxygenated, becoming Fe2+ (the oxygen molecules move to fill the vacancies). The presence of oxygen vacancies improved the conductivity of the photocatalyst electrode, at the same time giving it a significant surface potential gradient, thereby promoting the separation of electrons and holes.
At the same time a large amount of holes moved to the surface of the particles, allowing a high rate of oxygen evolution from water. The accumulation of holes improved the efficiency of the water oxidation reaction; the slow oxidation of the water has previously been a bottleneck in water-splitting. This enabled the researchers to achieve the world’s highest solar water-splitting performance for hematite anodes.
This strategy can be applied to a wide range of photocatalysts, beginning with solar water-splitting.
In addition to boosting the high efficiency of what is thought to be the world’s highest performing photoanode, this strategy will also be applied to artificial photosynthesis and solar water-splitting technologies via collaborations between the university and industries.
Previously, Tachikawa and his colleagues developed mesocrystal technology, which involves precisely aligning nanoparticles in photocatalysts to control the flow of electrons and their holes.
They produced the mesocrystal photoanodes by coating a transparent electrode substrate with hematite mesocrystals containing titanium and then annealing them at 700 ºC. A co-catalyst was deposited on the surface of the mesocrystals. When the photocatalysts were placed in an alkaline solution and illuminated with artificial sunlight, the water-splitting reaction took place at a photocurrent density of 5.5 mA cm-2 under an applied voltage of 1.23V.
The key to achieving a high conversion rate is the size of the nanoparticles that make up the mesocrystal structure. It is possible to greatly increase the amount of oxygen vacancies that form during the sintering process by making the nanoparticles as small as 5 nm and increasing the connecting interfaces between the nanoparticles. This boosted the electron density, and significantly increased the conductivity of the mesocrystals.
Next, the researchers will collaborate with industries to optimize the hematite mesocrystal photoanodes and implement an industrial system for producing hydrogen from solar light. At the same time, the strategy developed by this study will be applied to various reactions, including artificial photosynthesis.