
The global oil industry wastefully burns off about as much natural gas as is used by all of Central and South America – but a new methane binding agent offers a potential way for this greenhouse gas to be converted economically into liquid fuels.
Methane is a viciously potent greenhouse gas, if a relatively short-lived one. Over a 20-year span, methane emissions are estimated to have a global warming potential 84 to 87 times that of carbon dioxide. Global atmospheric methane concentrations were relatively stable for the last few hundred thousand years, but have increased rapidly since the 1750s and the beginning of the industrial revolution.
It comes from just about everywhere – to quote NASA Earth Observatory writer Adam Voiland, "You can find the odorless, transparent gas miles below Earth’s surface and miles above it. Methane bubbles up from swamps and rivers, belches from volcanoes, rises from wildfires, and seeps from the guts of cows and termites (where is it made by microbes). Human settlements are awash with the gas. Methane leaks silently from natural gas and oil wells and pipelines, as well as coal mines. It stews in landfills, sewage treatment plants, and rice paddies."
Oil production, according to the International Energy Agency (IEA), is currently responsible for about 40 percent of the methane emissions across the oil and gas industries. The methane that escapes during oil drilling is of no interest to oil companies; the economics of channeling it into natural gas infrastructure don't work out, it's dangerous and inconvenient to store, and while it can be catalytically converted into much more usable liquid fuels like methanol, the process has been too expensive to consider.
So they burn it, flaring it off into the sky. That's probably more environmentally responsible than just letting it rise into the atmosphere in the short term – but it results in some 265 million tons of carbon dioxide emissions, while doing no useful work for anyone.
As long as there are oil wells, this excess methane will need to be dealt with, and researchers at the University of New South Wales say they've made a crucial breakthrough in methane binding that opens the door to much more efficient, effective and hopefully affordable catalytic transformations.
The problem, until now, has been holding methane molecules still enough to study them. According to UNSW, researchers have been able to hold methane in a molecular "vice" using metal-methane complexes – but only for a few microseconds. That's nowhere near the few minutes it takes to properly analyze these kinds of molecules in a nuclear magnetic resonance spectroscopy device.
The UNSW team used computational modeling to attempt to predict whether other metals might bind methane for longer, and the results pointed to osmium – a platinum metal and the highest-density element that's found in nature. The team went ahead with experiments, and the results were impressive.
"We have found that methane, which is generally inert, will interact with an osmium-metal-centered species to form a relatively stable osmium-methane complex," says James Watson, lead author on a new study. "Our complex has an effective half-life of around 13 hours.”
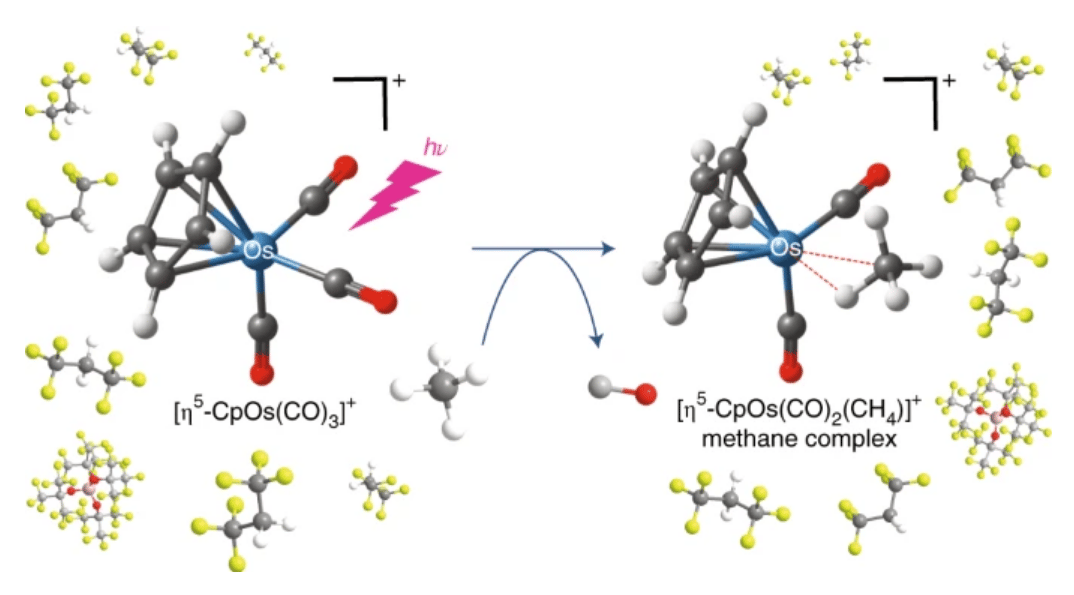
A new osmium-based complex has been found to bind methane extremely effectively, with a half-life around 13 hours as opposed to the microseconds previously possibleUNSW
“This means that it takes 13 hours for half of the complex to decompose," he continues. "This stability, in conjunction with the relatively long lifetime of this complex, allows for in-depth analysis of the structure, formation and reactivity of this class of [osmium] complexes and helps to inform the design of catalysts that have the potential to transform methane into more synthetically useful compounds.”
Osmium is one of Earth's rarest elements, but that's not likely to be a factor here. What's important is that, for the first time, these osmium complexes allow the kind of in-depth molecular analysis that should lead to new catalytic processes using much cheaper and more available elements that can quickly and economically convert methane into liquid fuels.
"One way of converting methane to liquid fuels is through the use of catalysts that contain transition metal elements,” explains study co-author Associate Professor Graham Ball. "Not only [are liquid fuels] far more convenient and far safer than storing gases, but also [come in] at a much lower energy cost."
“Liquid fuels are easier to transport, he continues, "and would be easily integrated into our existing fuel infrastructure – E10 petrol already has 10 per cent ethanol. If there were efficient, commercially viable methods to convert methane to methanol, for example, this would also provide an incentive to retain methane for conversion, and to avoid burning it without purpose, reducing overall fossil fuel use and damaging emissions. We hope that our discovery will inform the design of next-generation, more efficient catalysts that can be commercially viable."
Now, no liquid hydrocarbon fuel, including methanol, can be viewed as environmentally friendly, since burning it will result in carbon dioxide emissions. But if it's going to be burned, like the massive volume of methane that's currently being flared, it might as well do a job for somebody instead of being completely wasted. It's a transitional step in humanity's struggle to clean up our atmosphere, but it has the potential to make an enormous impact if further research proves as positive as the team expects it will.