A University of Michigan team has shown that a network of aramid nanofibers, recycled from Kevlar, can enable lithium-sulfur batteries to overcome their Achilles heel of cycle life, delivering an estimated 1,000 real-world cycles. A paper on their work is published in Nature Communications.
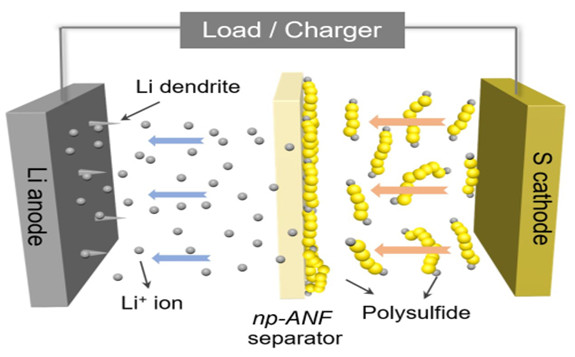
Previously, Kotov’s team had relied on networks of aramid nanofibers infused with an electrolyte gel to stop one of the main causes of short cycle-life: dendrites that grow from one electrode to the other, piercing the membrane. The toughness of aramid fibers stops the dendrites.
However, lithium sulfur batteries have another problem: small molecules of lithium and sulfur form and flow to the lithium, attaching themselves and reducing the battery's capacity. The membrane needed to allow lithium ions to flow from the lithium to the sulfur and back—and to block the lithium and lithium polysulfides. This ability is called ion selectivity.
The lithium ions and lithium polysulfides are similar in size, so it wasn’t enough to block the lithium polysulfides by making small channels. Mimicking pores in biological membranes, the U-M researchers added an electrical charge to the pores in the battery membrane.
They did this by harnessing the lithium polysulfides themselves: they stuck to the aramid nanofibers, and their negative charges repelled the lithium polysulfide ions that continued to form at the sulfur electrode. Positively charged lithium ions, however, could pass freely.
As a battery, Kotov says that the design is “nearly perfect,” with its capacity and efficiency approaching the theoretical limits. It can also handle the temperature extremes of automotive life, from the heat of charging in full sun to the chill of winter. However, the real-world cycle life may be shorter with fast charging, more like 1,000 cycles, he sayid. This is considered a ten-year lifespan.
Along with the higher capacity, lithium-sulfur batteries have sustainability advantages over other lithium-ion batteries. Sulfur is much more abundant than the cobalt of lithium-ion electrodes. In addition, the aramid fibers of the battery membrane can be recycled from old bulletproof vests.
The research was funded by the National Science Foundation and the Department of Defense. The team studied the membrane at the Michigan Center for Materials Characterization. The University of Michigan has patented the membrane and Kotov is developing a company to bring it to market.
Kotov is also the Joseph B. and Florence V. Cejka Professor of Engineering and a professor of chemical engineering, materials science and engineering, and macromolecular science and engineering.