Employees of Samara Polytech University, the specialists from the Department of Gas Processing, Hydrogen and Special Technologies and the Research Center "Fundamental Problems of Thermophysics and Mechanics," conducted theoretical and experimental studies of hydrodynamics, heat transfer and diffusion during methane pyrolysis (natural gas) in a layer of molten tin. The latest research results are published in the International Journal of Hydrogen Energy.
When methane is heated to high temperatures (over 1000 °C), it decomposes (pyrolysis process) into hydrogen and carbon nanoparticles with unique physicochemical properties. Among the existing methods of decomposition of methane, the most energy efficient is its heating during its transition through a layer of metal molts in reactors. This method is environmentally friendly, as it does not emit carbon dioxide into the atmosphere.
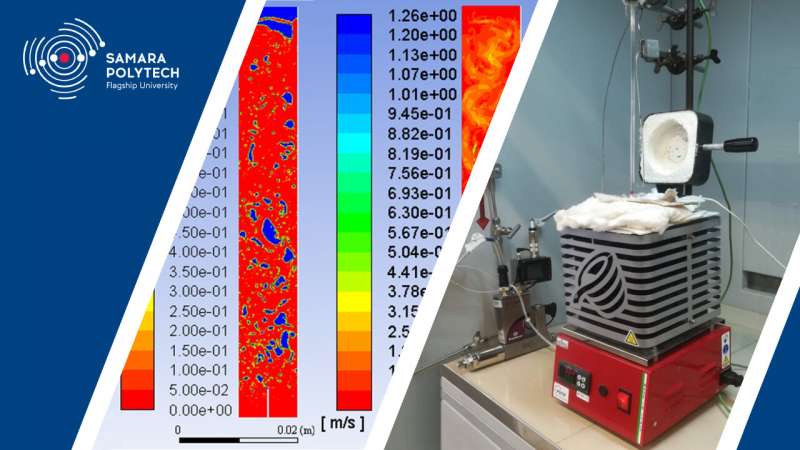
"The reactors are vertical-cylindrical structures filled with molten metal, in the lower part of which there is a nozzle for supplying methane," Igor Kudinov, Doctor of Technical Sciences, Professor, Director of Research Center "Fundamental Problems of Thermophysics and Mechanics" explains. "When designing a reactor, it is necessary to pay special attention to its height and volume, so that methane during its transition through a layer of molten metal, heats up to the pyrolysis temperature and completely decomposes. In this case, it is necessary to determine the concentration and speed of movement of methane, as well as the temperature and pressure of the mixture of methane with tin over the entire height of the reactor. Thus, it is necessary to solve the problem of interconnected heat and mass transfer.
In addition, the staff of the department of Gas Processing, Hydrogen and Special Technologies carried out an experimental study of methane pyrolysis through a layer of molten tin on a specially made experimental stand.
"During the experiment, a large amount of soot was formed on the surface of the tin, which could not be removed from the reactor crucible together with the gas discharged from the facility," Andrey Pimenov, Doctor of Technical Sciences, Professor, Head of the Department of Gas Processing, Hydrogen and Special Technologies noted. "We have developed a special device that controls the level of molten metal in the reactor and continuously removes solid carbon particles."