Fluorides have been identified as a key ingredient in interphases supporting aggressive battery chemistries. While the precursor for these fluorides must be pre-stored in electrolyte components and only delivered at extreme potentials, the chemical source of fluorine so far has been confined to either negatively-charge anions or fluorinated molecules, whose presence in the inner-Helmholtz layer of electrodes, and consequently their contribution to the interphasial chemistry, is restricted.
To pre-store fluorine source on positive-charged species, here we show a cation that carries fluorine in its structure is synthesized and its contribution to interphasial chemistry is explored for the very first time.
An electrolyte carrying fluorine in both cation and anion brings unprecedented interphasial chemistries that translate into superior battery performance of a lithium-metal battery, including high Coulombic efficiency of up to 99.98%, and Li0-dendrite prevention for 900 hours. The significance of this fluorinated cation undoubtedly extends to other advanced battery systems beyond lithium, all of which universally require kinetic protection of highly fluorinated interphases.
—Liu et al.
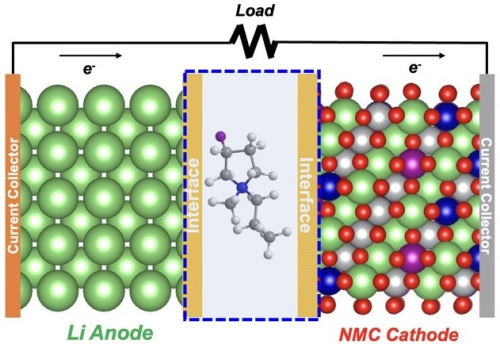
Design of lithium metal battery with electrolyte containing a fluorinated cation (atomic structure at center). The interface area represents the layer with fluorine that forms on the anode surface, as well as the cathode surface. (Image by Argonne National Laboratory.)
Lithium metal batteries with our fluorinated cation electrolyte could considerably boost the electric vehicle industry, and the usefulness of this electrolyte undoubtedly extends to other types of advanced battery systems beyond lithium ion.
—Zhengcheng (John) Zhang, co-corresponding author
Although lithium-metal batteries can deliver more than double the energy density possible with a lithium-ion battery, that outstanding performance rapidly vanishes within less than a hundred charge-discharge cycles.
In lithium metal batteries, the electrolyte is a liquid consisting of a lithium-containing salt dissolved in a solvent. The source of the short cycle-life problem is that the electrolyte does not form an adequate protective layer on the anode surface during the first few cycles. This layer, also called solid-electrolyte-interphase (SEI), acts like a guardian, allowing lithium ions to freely pass in and out of the anode to charge and discharge the battery, respectively.
The team discovered a new fluoride solvent that maintains a robust protective layer for hundreds of cycles. It couples a fluorinated component that is positively charged (cation) with a different fluorinated component that is negatively charged (anion). This combination is called an ionic liquid—a liquid consisting of positive and negative ions.
The key difference in our new electrolyte is the substitution of fluorine for hydrogen atoms in the ring-like structure of the cation part of the ionic liquid. This made all the difference in maintaining high performance for hundreds of cycles in a test lithium metal cell.
—Zhengcheng (John) Zhang
To better understand the mechanism behind this difference at the atomic scale, the team drew upon the high performance computing resources of the Argonne Leadership Computing Facility (ALCF), a DOE Office of Science user facility.
Simulations on the ALCF’s Theta supercomputer revealed that the fluorine cations stick to and accumulate on the anode and cathode surfaces before any charge-discharge cycling. Then, during the early stages of cycling, a resilient SEI layer forms that is superior to what is possible with previous electrolytes.
High-resolution electron microscopy at Argonne and Pacific Northwest National Laboratory revealed that the highly protective SEI layer on the anode and cathode led to the stable cycling.
The team was able to tune the proportion of fluoride solvent to lithium salt to create a layer with optimal properties, including an SEI thickness that is not too thick or thin. Because of this layer, lithium ions could efficiently flow in and out of the electrodes during charge and discharge for hundreds of cycles.
The team’s new electrolyte offers many other advantages as well. It is low cost because it can be made with extremely high purity and yield in one simple step rather than multiple steps. It is environmentally friendly because it uses much less solvent, which is volatile and can release contaminants into the environment. And it is safer because it is not flammable.
This work was supported by the DOE Office of Energy Efficiency and Renewable Energy, Vehicle Technologies Office. Computing time on the ALCF was awarded through DOE’s ASCR Leadership Computing Challenge.