Image Credit: VectorMine/Shutterstock.com
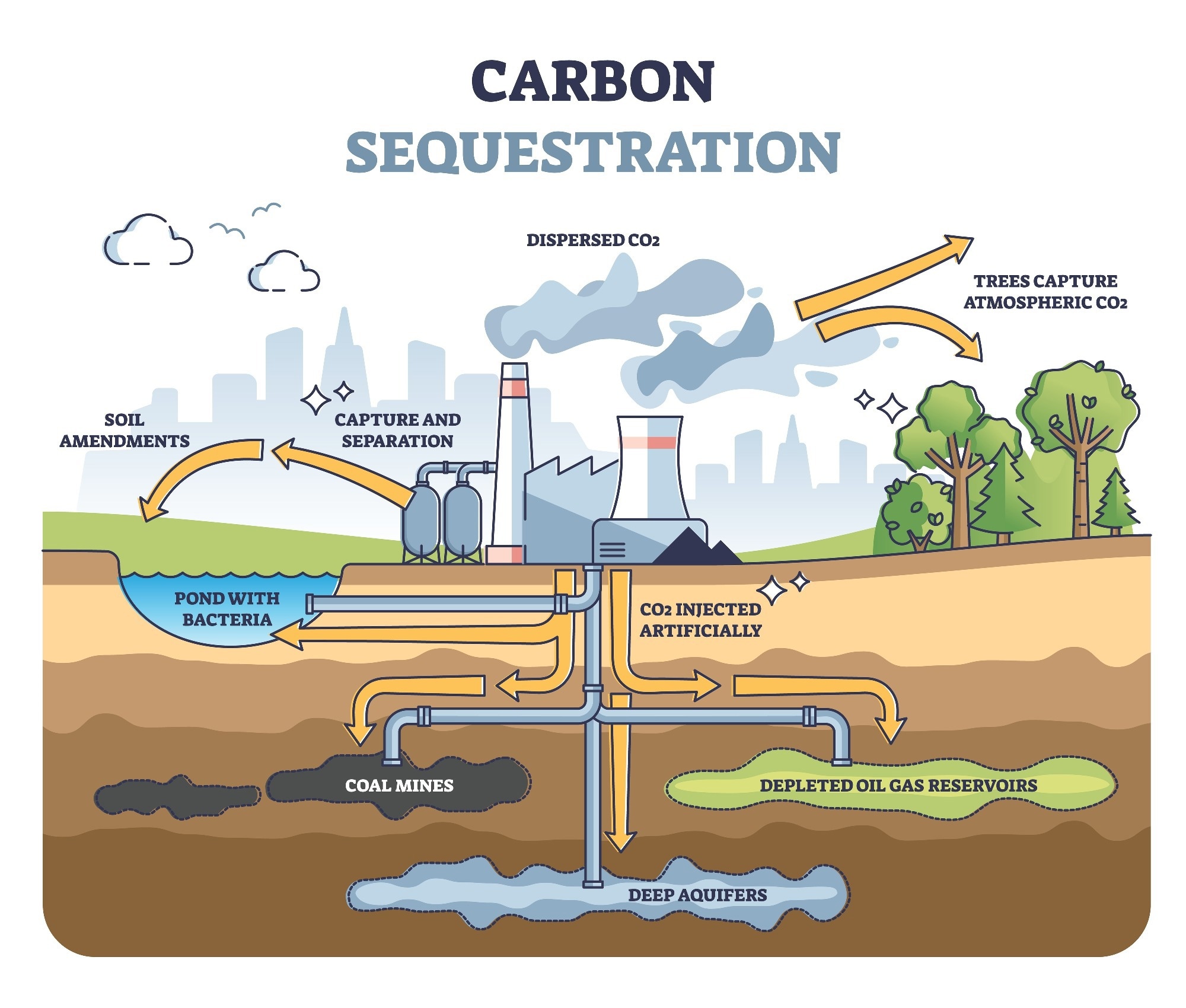
Countries are feeling the pressure to meet net-zero goals, and carbon capture and storage technologies could be a big help. The International Energy Agency estimated that 100 billion tons of CO2 must be stored by 2060 to avoid a harmful global temperature rise to 2 degrees Celsius. The CO2 can be stored in former oil or gas reservoirs or even coal seams or salt aquifers such as those below the seabed of the North Sea.
The CCUS technology pressurizes the CO2 and makes it a liquid form called 'supercritical CO2’. It is then transported via a pipeline and injected at depth into rock formations. Primarily, the permeability of the rock storing the CO2 is important.
A 'cap rock' usually describes an impermeable layer that acts as a lid so that liquids and gases cannot rise and escape. In the context of carbon sequestration, this is called 'structural storage', which is the same mechanism in which oil and gas have stayed underground for millions of years.
To make the storage more permanent, the trapped CO2 will begin to react with the rock it is stored in. As solid minerals are subsequently formed, this helps seal in the CO2 (mineral storage).
In saline aquifers, the CO2 also descends below the aquifer, leading to 'dissolution storage'. Even though leakage is possible, research suggests it will be minimal, a study in Nature speculated that more than 98% of injected CO2 will remain in storage for over 10,000 years. While that is promising, and there are industrial-scale carbon storage projects taking place in North America, UAE, and Australia, there are still big steps to take to decarbonize entire industries.
Can Sequestered Carbon Escape?
Carbon stored underground could find multiple ways to escape and seep back into the atmosphere due to chemical reactions that take place between the carbon dioxide, rocks, water in the pores and even the cement from abandoned wells, according to researchers from Penn State University.
If these emissions escape, this would add to existing greenhouse gas emissions which exacerbate global warming.
The research team became aware of this risk when they investigated the rock properties where CO2 is injected into limestone and sandstone, which present different chemical properties.
Li Li, associate professor of petroleum and natural gas engineering said: “We were interested in examining these rocks because they are widely found underground, but there have been concerns that carbon dioxide may escape once it’s injected underground.”
“Even if it doesn’t escape to Earth’s surface, there are concerns that it may leak into groundwater drinking aquifers,” Li Li added.
The CO2 could increase the acidity of the saltwater and lead to the dissolving of some rocks, particularly those high in calcium carbonates such as limestone, as well as the cement linings of abandoned oil and gas wells.
As a result of the interaction between the CO2-infused brine reaching an abandoned well, it can lead to cracks in the cement that could cause the CO2 to escape.
A research team, comprising Zuleima Karpyn, associate professor of petroleum and natural gas engineering, and Quentin E. and Louise L. Wood Faculty Fellow in Petroleum and Natural Gas Engineering, has investigated what happens when brine interacts with rocks such as limestone. They recreated what could happen chemically underground and to the rock layers, with results published in the International Journal of Greenhouse Gas Control.
Results showed that the brine dissolved parts of the limestone, making it more permeable, allowing liquids and gases to move through it more easily, rendering more escape pathways. Meanwhile, the brine became less acidic, so it did not dissolve any cement that would be located in an abandoned well. This means that the rock itself was the main medium to react, and the cement was secondary. Therefore, wellbores are more likely to stay intact in limestone formations. However, the limestone can be dissolved and create leakage pathways. This does not apply to all rocks. For sandstone, the team’s simulation revealed that it was the other way around, where the brine dissolved the cement more than the sandstone, which lost very little mass.
To achieve carbon sequestration in rock layers underground, teams need to consider the geochemical that can take place, depending on the rock type and proximity to a cemented wellbore. Finding ideal sites for injection considers more than just the rock properties and geochemistry but also the permeability and way in which water and CO2 may flow through these rocks.